- Get link
- X
- Other Apps
Can artificial cells be built from basic components? Systems that have complex architectures and functions evocative of natural cells have been prepared by recycling the contents of bacterial cells in synthetic droplets.
- N. Amy Yewdall
Global collaborations have made promising strides towards building artificial cells ‘bottom up’ from constituent parts1, but the current state-of-the art results are still far from resembling anything alive. The difficulty lies in organizing functional features, such as enzymes, at the nanoscale and ensuring network connectivity between all the components. Writing in Nature, Xu et al.2 report that they have tackled this challenge by organizing pieces of broken-down cells on a synthetic scaffold, thereby producing artificial cells with functional and compositional complexity reminiscent of living ones. The findings take us a step closer to life-like systems that could be used for industrial applications — and towards a better understanding of life itself.
Xu et al. use compartments known as coacervates to make the synthetic scaffold for their artificial cells. Coacervates are membrane-free droplets made of a dense liquid phase that form and separate spontaneously from aqueous solutions — a process known as liquid–liquid phase separation. The authors’ coacervates form through the associative interactions between a synthetic polymer and a nucleotide molecule, ATP.
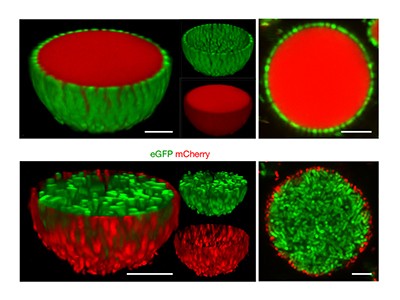
The authors use this platform to capture, and then rupture, a mixture of two types of bacterium, thereby generating artificial cells consisting of a coacervate with an outer membrane and internal subcompartments (Fig. 1). The ruptured bacterial cells release their contents of proteins and metabolite molecules, most of which are retained in the dense coacervate core. This creative method results in the localization of active enzymes inside the ‘cytoplasm’ of the artificial cell. The authors also demonstrate that the coacervate core has the ability to make small quantities of proteins — that is, it carries out in vitro transcription and translation (IVTT) — using the protein-synthesis machinery released from the bacteria.
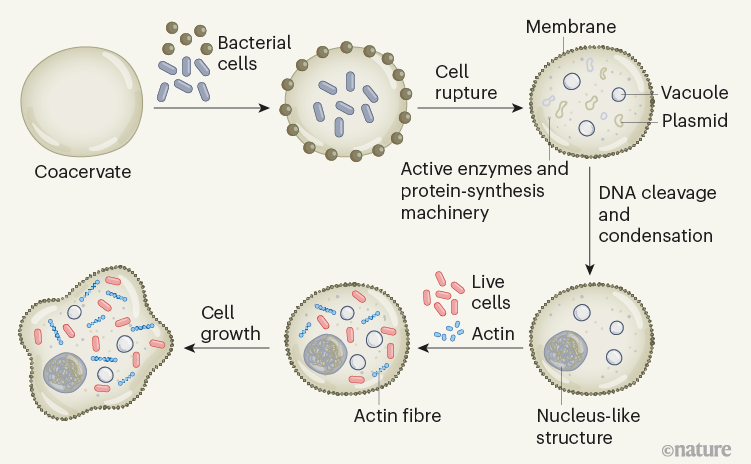
Figure 1 | Construction of an artificial cell. Xu et al.2 introduced two types of bacterium into membrane-free droplets, known as coacervates. One type localized inside the droplets and the other on the droplets’ surface. The authors ruptured the bacteria, releasing the cell components. This resulted in the formation of an artificial cell with a bacterium-derived membrane, surrounding a coacervate core that contains active enzymes, functional protein-synthesis machinery, water-filled chambers (vacuoles) and circular bacterial DNA (plasmids). Enzymatic cleavage of the plasmids into short strands enabled the DNA to be condensed into a nucleus-like structure. Actin proteins could be introduced, which assembled into fibres, providing a rudimentary cytoskeleton. The addition of live bacterial cells resulted in the artificial cell’s morphing into an amoeba-like shape.
Artificial cells have previously been made from living cells or purified cell-derived components3, but none of those involved such a direct approach as Xu and colleagues’ strategy. The authors’ work suggests the thrilling potential of using coacervates as platforms to host and harness a variety of cells, connecting their functionality in original ways. For example, coacervates could combine the components of mixtures of bacteria that contain different types and concentrations of enzymes, for synthetic biology applications.
Read the paper: Living material assembly of bacteriogenic protocells
How do these artificial cells differ from actual cells? In the current work, essentially the whole artificial cell is a coacervate, whereas liquid–liquid phase separation is responsible only for the formation of subcellular compartments — called biomolecular condensates — in natural cells4. Nevertheless, Xu et al. show that subcellular organization can also be achieved in their system. The authors use an enzyme to cleave bacterial DNA into short strands in the ‘cytoplasm’ of their artificial cells, and then add a negatively charged polymer and histones (the proteins with which DNA is associated in the nucleus), which causes the DNA to condense into a nucleus-like structure. This type of hierarchical organization, in which a condensed phase forms inside another, had previously been observed in multiphase coacervates5,6, and hints at how DNA might be compartmentalized in living cells7.
The DNA core in Xu and colleagues’ artificial cells is currently only a structural feature, and so the next challenge will be to use it for a specific function. The possibilities are endless. With some optimization, one could imagine the sequestered DNA serving as a useful starting point for gene circuits or IVTT reactions, for example.
Living cells divide to reproduce, which requires each cell to change shape before splitting into two. Much work is going on to achieve the division of artificial cells consisting of membrane-bound vesicles, with many research groups investigating the use of proteins from the cytoskeleton (see refs 8–10, for example) — the network of structural filaments that gives a cell its shape. However, it has proved more difficult to change the shape of coacervates11.
First complete model of the human embryo
Xu et al. show that actin, a cytoskeleton protein, can be polymerized enzymatically in the ‘cytoplasm’ of their artificial cells to produce a filamentous network. However, the overall morphology of the artificial cells remained spherical during this process. Intriguingly, the authors then encapsulated living bacteria inside the coacervates, and observed that the artificial cells morphed over time into an asymmetrical shape, reminiscent of that of natural cells such as amoeba. Although visually exciting, it is hard to see for now how this shape change might lead the way to artificial cell division.
A major drawback of Xu and colleagues’ seemingly straightforward approach for using living systems as the basis for artificial cells is that all life forms contain many unknowns. Even the genome of the ‘minimal cell’ reported12 in 2016 — which was engineered to contain only the genes thought to be essential for life — has a substantial fraction (about 30%) whose function is unknown, and bacterial cells are more complex than that. To build a fully controllable artificial cell, it will be necessary to identify the constituent parts needed and to understand how they interact with each other. For now, this work intriguingly demonstrates that biochemistry can function in conditions different from those found in natural cells.
The combinations of living and artificial cells reported by Xu et al. should make us all ponder, ‘what is life?’ Scientists typically use a reductionist approach that defines living things as those that have specific characteristics — such as cellular and subcellular compartmentalization, metabolism, information storage and processing, and regenerative capabilities13. Several types of artificial cell have emulated a few of these hallmarks14. However, if life is an emergent property that arises from complex network systems15, then artificial cells must be able to integrate and connect many more of the characteristics just mentioned.
Xu and colleagues have provided a platform that might achieve this. Their current system integrates seven attributes of living cells: an outer membrane; a crowded interior; the ability to carry out cascades of enzymatic reactions; protein-synthesis capabilities; impressive structural organization with diverse subcompartments; a primitive cytoskeleton; and the ability to adopt asymmetric cellular shapes. But a truly living system has yet to emerge from the test tube — the reported artificial cells are the equivalent of cell-like automatons. Nevertheless, the new findings are an important step forwards in this field, demonstrating the power of coacervates to localize and integrate diverse biomaterials, including living cells, to make artificial cells. The next challenge is to make progressively interconnected networks that close the gap between artificial and living cells.
doi: https://doi.org/10.1038/d41586-022-02231-8
- Get link
- X
- Other Apps

Comments
Post a Comment